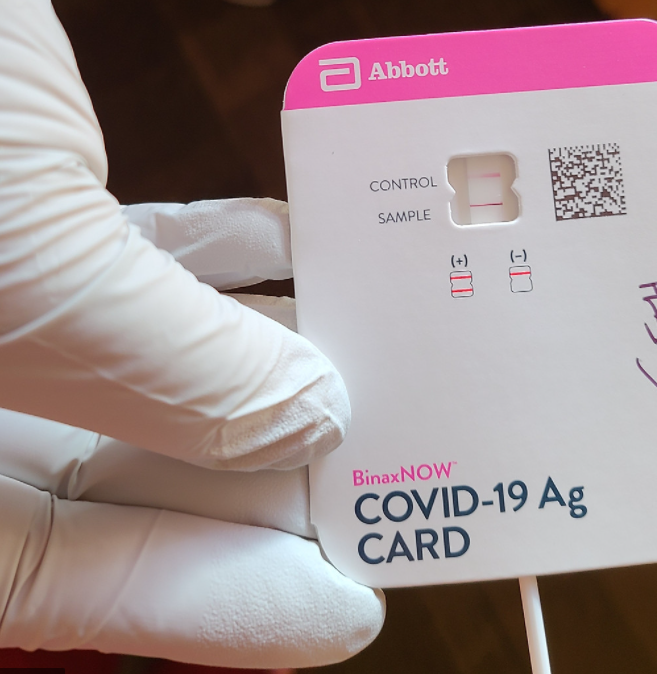
Description
INTENDED USE
TheBinaxNOWTM COVID-19 Ag Card is a lateral flow immunoassay intended for the qualitative
detection of nucleocapsid protein antigen from SARS-CoV-2 in direct anterior nasal (nares) swabs
from individuals suspected of COVID-19 by their healthcare provider within the first seven days
of symptom onset. Testing is limited to laboratories certified under the Clinical Laboratory
Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to
perform moderate, high or waived complexity tests. This test is authorized for use at the Point of
Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate
of Compliance, or Certificate of Accreditation.
The BinaxNOWTM COVID-19 Ag Card does not differentiate between SARS-CoV and SARS-CoV2.